B-HIVE Research Highlight: Transcription start sites
One sequence, two functions
As HIV-1 replicates, unspliced RNA plays two very different functions. It’s packaged into the genome of viral particles, and is translated to form proteins essential for virus assembly. Previous studies found that the virus differentiates between these two functions of unspliced RNA, which are virtually identical, by the number of guanosines at the 5’ end of the transcript. By shifting the transcription start site, HIV-1 produces unspliced RNA with slightly different 5’ sequences. RNA species beginning with one guanosine (1G) and three guanosines (3G) are the most prevalent. 1G RNA is incorporated into new virions as the genome, while 3G RNA is translated to produce Gag/Gag-Pol polyproteins required to assembly new virions. Cell culture experiments established the distinct functions of these species, which differ by only two bases. But, because the in vivo human cell environment is much more complex than cell cultures, B-HIVE researchers asked – is this same transcription site selection happening in human cells?
Unspliced RNA in the “real world”
With same-day samples of blood cells and plasma from three people, as well as plasma samples from eight additional people, the researchers studied the viral unspliced RNA in both types of samples. RNA in cell samples would show the species produced in infected cells, and RNA in plasma samples would show the preferred species packaged into virions.
The results showed that, in cell samples, 3G RNA was the dominant species. Plasma samples showed a different trend, with 1G RNA overwhelmingly preferred.
Why would the virus care about one RNA species versus another? Previous B-HIVE research showed that each has its advantages: 1G RNA folds differently than 3G RNA. Its folded structures expose key binding sites, making it a better choice as a component of the viral genome. 3G RNA produces more translated protein than 1G RNA, making it a better choice as a translation template. Other studies showed that mutations knocking out production of either 1G or 3G RNA hinder virus replication, suggesting that employing both RNA types in their respective roles confers a replication advantage.
What this tells us about HIV-1
The results show that the unspliced RNA transcription processes observed in cell cultures are indeed occurring in human systems. The study suggests that HIV-1 takes advantage of the ability of host RNA polymerase II to initiate transcription at multiple neighboring sites to generate multiple advantageous unspliced RNA species.
More research is needed to fully understand the roles of multiple species of unspliced RNA in HIV-1 and other retroviruses. But confirming that utilizing multiple transcription sites is an important process in maintaining viral replication fitness in vivo suggests that 3G and 1G RNA may be targets for disrupting HIV-1’s replication signaling pathways.
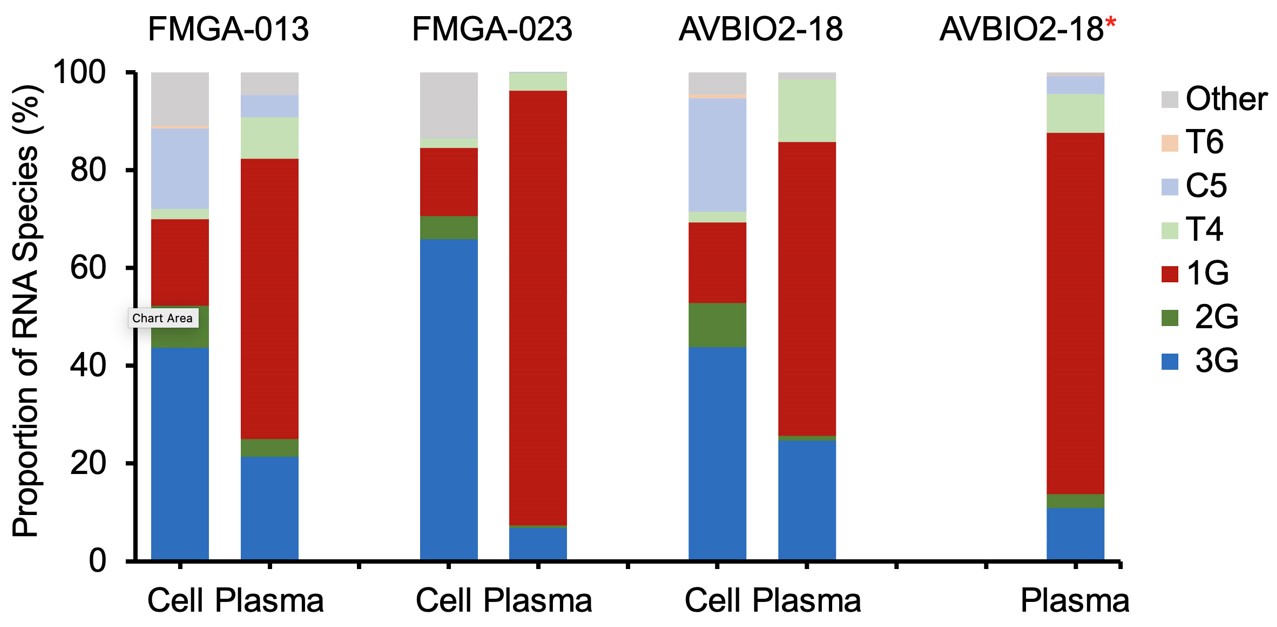
Distribution of HIV-1 RNA species in study participants. The proportion of HIV-1 RNA species in Peripheral Blood Mononuclear Cells (PBMCs) and plasma collected from same study participants at the same time. AVBIO2-18* is the only plasma RNA sample collected 3-months apart from the PBMC sample collection.
Meet the Researcher
How did you get interested in science?
Ever since I was a kid, I’ve always been interested in animals, nature, in flowers, plants, living beings, wondering how they grow and so on. So I don’t think I even understand what science was at that time. Those were the things that piqued my interest.
I was trained as a veterinarian. When I finished my veterinarian program, I decided that I didn’t learn enough. There were still so many things I didn’t understand. I was wishing that I could come back and treat all the sick animals and make them healthy. But I really didn’t understand most of the time what made them sick. So, I decided to study other things. I walked into a molecular biology and virology lab, and that was that!
Tell us about the lab where you did this work.
It’s a very collaborative project. A large part of the study is done by one postdoc fellow with help from another postdoc fellow and a research scientist in my lab who developed an assay. This is done in collaboration with three other PIs, including Frank Maldarelli, who runs clinical trials and has access to clinical samples. So, when we discussed this question with him, he’s the one who identified patient samples. That sounds very easy. But it’s not that simple because he had to figure out what kind of things we were looking for, and what kind of sample might fit our needs. He had to find samples from multiple clinical trials for these studies. Two other groups also helped us with this study.
What were the biggest challenges with this study?
In a cell culture system, we can control how much virus is there. We have plenty of material for our analysis. But with clinical samples, you’ve got what you got! In general, in cell culture systems we have far more RNA copies that we have in clinical samples.
So one of the first things we did is take our assay for the cell culture system, titrate it down and see how sensitive it is. How low can we go? And then we said, “Okay, this is how much RNA we need.” Then we had to find the samples. A lot of the clinical samples are from people who are on antiviral treatment. So in these people their viral load is very low, which is great, but we need a certain amount of virus to do the assay we’re doing. So, we had to go back to samples from people who were not currently on antivirals. Those tend to be older samples that have been in the freezer for many, many years. If that’s the case, you don’t always find samples from the same person that had both the cell and the virus collected the same day. That’s really hard, too. So we actually showed three pairs with one extra sample.
But we actually went through 42 samples before we could identify our final data set. Oftentimes we did the assay and it turned out that there wasn’t enough RNA. It was not that easy to find all these samples. It’s actually much harder than it sounds.
What are you working on now?
We identified HIV as a pathogen more than 40 years ago. But at every turn, there is a surprise about how this pathogen takes advantage of the host to make the host do what the virus wants. HIV regulates everything in very, very intricate ways. And there’s a lot of that regulatory mechanism we do not understand. One big surprise for me was how the virus can tease things out and make these two RNAs, one good for this, one good for that, to optimize its replication. That was truly astonishing.
Knowing what the virus can do now, we’re looking forward to finding out more about how the virus regulates replication. That’s really what we want to know, because if you know how regulation works, then you can think about countermeasures. But if you don’t know how regulation works, it’s a bit of a shot in the dark.
We are collaborating with multiple B-HIVE labs. For example, the paper that figured out that the virus really needs both RNAs to replicate well was in collaboration with four other B-HIVE members. So it’s truly a team effort.

