B-HIVE Research Highlight: Lenacapavir’s effect on capsid integrity
Unveiling unknown mechanisms
Lenacapavir is the first in a new class of drugs to treat multi-drug resistant HIV-1. It is classified as a capsid inhibitor, although the precise mechanism of how lenacapavir affects the capsid—the lattice of proteins that encases the viral core until penetration of the nucleus—was unclear. Understanding the mechanism of HIV-1 inhibition by lenacapavir is important to developing next-generation drugs, as well as studying the mechanisms by which the virus may develop resistance to the drug.
B-HIVE researchers previously developed methods to fluorescently label the capsid and the viral core separately. These methods led to insights in the timing of when the viral core uncoats and sheds the capsid within the nucleus, shortly after nuclear entry and near the integration site. Here, they fluorescently labeled the capsid and core separately to observe how lenacapavir and a less potent capsid inhibitor, PF74, affect the capsid and core through the process of nuclear entry.
Labeling the capsid and core
The researchers fused green fluorescent protein, or GFP, to the capsid, which served as a marker of capsid stability. They separately introduced a fluid-phase GFP marker, which labeled the viral cores. These markers showed that the viral cores disappeared after lenacapavir treatment while the capsid remained stable. Loss of the fluid-phase GFP markers after lenacapavir treatment suggests that the drug compromises the capsid just enough to cause a loss of viral core integrity.
Although the lenacapavir-treated cores were stabilized, the researchers found that the drug breaks the capsid shell in a way that the capsid can still dock with the nuclear pore but cannot successfully import into the nucleus. In contrast, treatment with PF74 led to a loss of GFP markers on the capsid, suggesting destabilization of the capsid lattice leading to disintegration of the viral cores. In this way the mechanism of HIV inhibition is different in PF74 in that the capsid disassembles before it has a chance to dock with the nucleus.
What this tells us about HIV-1
By elucidating the mechanism of lenacapavir capsid inhibition, the study further reveals the importance of an intact capsid for HIV-1 to enter the nucleus. Interfering with nuclear import, an essential step in viral replication, helps lenacapavir treat multidrug-resistant HIV-1. Further, the study shows how even broken yet stable capsids are able to dock with nuclear pore complexes, adding to understanding of the docking and nuclear import process.
Previous studies of lenacapavir-treated capsids used a negative stain transmission electron microscopy method to assess the integrity of the capsids. These previous methods showed intact capsids. The results of this study show that the capsids were indeed broken yet stable, suggesting that breaks not detectable by negative stain TEM may still be significant for the viability of the viral core.
The insights gained from this study show the way for developers of next-generation capsid inhibitors. For instance, the results show that inhibiting import of the virus into the nucleus does not necessarily require rupturing the capsid structure, but disrupting it enough to compromise the viral cores. They also show how breaking the capsid impedes import of viral cores into the nucleus, even if the core remains intact.
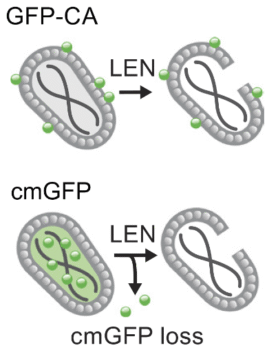
A schematic showing the effect of lenacapacir (LEN)-induced disruption of HIV-1 mature cores labeled with fluorescent markers on the capsid (GFP-CA, top) or in the fluid phase (cmGFP, bottom).
Meet the Researcher
How did you get interested in science?
I did a research project as an undergraduate student in the Department of Biology at the University of California, Los Angeles, and then another project as a summer student. These experiences showed me that I love the intellectual challenge of doing research and learning things that were previously unknown. I went to the University of California, Davis, where I received my Ph.D. in the Department of Genetics. I worked on translational initiation factors in John Hershey’s lab. Subsequently, I did a post-doctoral fellowship under the guidance of Nobel Laureate Howard Temin at the University of Wisconsin at Madison. I then went to West Virginia University as an Assistant Professor and was promoted with tenure to Associate Professor. In 1999, I joined the HIV Drug Resistance Program at the National Cancer Institute.
Tell us about the lab where you did this work.
This work was done in my lab, the Viral Mutation Section of the HIV Dynamics and Replication Program, at the National Cancer Institute by two very talented scientists: Chenglei Li and Ryan C. Burdick. Ryan Burdick played a central role in developing the method for fluorescent labeling of HIV-1 capsids with a GFP-CA fusion protein. Chenglei Li played a critical role in adapting another method to label HIV-1 cores with a fluid-phase content marker GFP, that we refer to as cmGFP. Our ability to fluorescently label the capsid shell with GFP-CA and to label the fluid phase of the viral cores with cmGFP were crucial tools that helped us to gain insights into the effects of lenacapavir on isolated HIV-1 cores as well as HIV-1 cores in infected cells.
What were the biggest challenges with this study?
We did some careful characterization of our two fluorescent labeling methods of HIV-1 cores to ensure that our interpretation of the cell-based assays was fully supported by the evidence. Although we had no doubts about our HIV-1 core labeling methods, it was important to provide further characterization to convince the field. Ultimately, these experiments helped to strengthen these tools for studies of HIV-1 biology, and we hope that they are used widely by others. Another challenge was to obtain electron microscopy analysis of HIV-1 cores that were treated with capsid inhibitors to gain insights into the effects of lenacapavir and PF74 on the structure of HIV-1 cores. Chenglei Li used his prior experience in electron microscopy, and in collaboration with Ru-Ching Hsia, obtained robust data on the structures of hundreds of HIV-1 cores that were treated with lenacapavir or PF74. The effort paid off, as our data showed that the lenacapavir treatment leads to more frequent breaks at the narrow end of the conical capsid shell.
What are you working on now?
Ryan Burdick has developed a new dual-labeling method so that we can examine the viral core integrity and capsid lattice for individual viral cores in infected cells. We are using this method to gain insight into the intranuclear locations at which viral cores uncoat. In addition, Ryan has determined that treatment of infected cells with capsid inhibitors leads to changes in the selection of the sites at which HIV-1 DNA integrates into the host genome. These studies are providing valuable insights into the spatiotemporal relationship between viral core uncoating and the selection of chromosomal sites of integration.

